Onset: mainly after adolescence
Site: generally appear on the face
Origin: Nests and cords of nevus cells are found within the dermis; they may extend into the subcutaneous fat
Size: vary in size from a few millimeters to a centimeter
Shape: Dome-shaped lesions are the most common, The variety of shapes reflects the evolutionary process in which moles extend downward with age and nevus cells degenerate or become replaced by fat and fibrous tissue
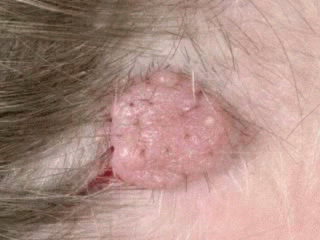
Appearance: elevated, fleshy, and slightly or moderately pigmented papules, may appear as a soft, flabby, wrinkled sack
Surface: smooth, may be white or translucent, with telangiectatic vessels
Color: brown or black, but may become lighter or flesh-colored with time
Border: symmetric, white borders may appear, creating a halo nevus
Special features: Pigmentation may be arranged in flecks. Coarse, dark, terminal hairs may grow from the nevus. may be warty or polypoid. Pedunculated lesions with a narrow stalk are located on the trunk, neck, axilla, and groin
Complications: prone to trauma from clothing and other stimuli, often causing them to bleed
Progress: Degeneration into melanoma is rare
Differential Diagnosis: 1- dermal nevi may resemble nodular melanoma, 2- trauma causing them to bleed and inflame, influencing some patients to suspect malignancy. 3- telangiectatic vessels on the surface mimicking basal cell carcinoma.

Histologically Dermal Nevi are composed of nests and cords of nevus cells are found within the dermis; they may extend into the subcutaneous fat. Melanocytic cells are pale, uniform in size and are found in cords or clusters surrounded by collagen bundles in the dermis.






Wednesday, June 25, 2008
 What are Dermal Nevi
What are Dermal Nevi
 What are Compound Nevi
What are Compound Nevi
Origin: both at the dermoepidermal junction and within the dermis
Appearance: slightly or markedly raised pigmented papules
Surface: smooth or slightly papillomatous
Border: have an irregular border but are symmetric
Special features: Hair may be present, the center tends to be more heavily pigmented than the periphery, If a white halo appears at the periphery of the lesion, it is referred to as a halo nevus
Progress: tend to increase in thickness and pigmentation in late childhood and adolescence.





Tuesday, June 24, 2008
 What are Junctional Nevi
What are Junctional Nevi
A junctional nevus is a mole found in the junction (border) between the epidermis and dermis layers of the skin.
 Nests of nevus cells cluster at the dermoepidermal junction
Nests of nevus cells cluster at the dermoepidermal junction
 Onset: rare at birth and generally develop after the age of 2 years
Onset: rare at birth and generally develop after the age of 2 years
Site: palms, soles, and the genitalia
Prevalence: most common in children
Size: vary from 0.1 to 0.6 cm
Appearance: flat or slightly raised brown to tan macules with uniform pigmentation
Surface: smooth and flat to slightly elevated
Border: round or oval and symmetric
Special features: Hairless, with preserved surface skin markings
Progress: may change into compound nevi after childhood, Degeneration into melanoma is rare.
Sunday, June 22, 2008
 Melanocytic Nevi Pictures
Melanocytic Nevi Pictures
Nevi or moles are defined as benign skin tumors. Histologically they are composed of melanocyte-derived nevus cells. Melanocytes are derived from neural crest tissue. According to age of onset and the location and arrangement of nevus cells within the skin, melanocytic nevi are classified into different types. Melanocytic nevi are arranged as organized clusters of nevus cells located at various levels in the skin. Nevi are equally common among males and females. The average number in adults is between 12 and 20 nevi. In some families a larger number of nevi may be encountered. Irritation by clothing or external trauma may be associated with the development of inflammatory symptoms.
Congenital Nevi
Nevi present at birth or that appear during infancy are termed congenital nevi.
Acquired Nevi
1- The appearance of newly acquired nevi reaches a peak during adolescence.
2- Fewer nevi are acquired after age 30, Nevi appearing after age 30 should be regarded as suspicious.
3- Sun exposure appears to be a stimulus for cell growth of nevi as most acquired nevi appear on sun-exposed skin. Acquired Nevi appearing appearing in sun-protected areas should also be regarded as suspicious.
4- Existing nevi may increase in size and become more heavily pigmented during puberty or during pregnancy.
Development of Nevi
Acquired nevi first appear as flat, round, uniformly colored papules. During this growth phase, nevi expand laterally while remaining flat and symmetric. Nevi may be slightly darker in color and slightly raised in the center, and may remain stable in size and appearance for several years. Over many years, nevi continue to become more elevated and uniformly lighter in color. Eventually the nevus appears as a skin-colored papule or may completely disappear in older years. Residual nevi are rare after age 70.


















Thursday, June 19, 2008
 How to prevent skin cancer
How to prevent skin cancer
SKIN CANCER PREVENTION
For the nonmelanoma skin cancers, the most logical approach to prevention is to limit exposure of the skin to natural and artificial sources of UV radiation. This can be achieved in numerous ways, including the use of sunscreens, the avoidance of outdoor activities during the noon hours when the amount of UV radiation in sunlight is maximal, and the use of protective clothing. Reducing the lifetime dose of UV radiation reduces the risk of skin cancer development. Because the effects of UV radiation in causing nonmelanoma skin cancers are cumulative, we would expect that reducing sunlight exposure at any age would retard the rate of tumor development.
For melanoma and perhaps even for some basal cell carcinomas, it is not clear whether this strategy would be effective because there is not a simple, direct relationship between dose of UV radiation and melanoma induction. For example, if melanoma results from childhood exposure to UV radiation, as has been suggested, reducing sunlight exposure during adult life may not be beneficial in attempting to decrease the incidence of melanoma. Obviously, more information on the dose response, wavelength dependence, and mechanism of action of UV radiation in the induction of melanomas is needed to devise effective strategies for preventing even the melanomas that are sunlight-related.
Saturday, June 7, 2008
 Skin cancer causes
Skin cancer causes
Risk factors for basal and squamous cell skin cancers are strikingly similar. These lesions, although seen in younger age groups, are most often encountered in patients 60 years of age or older.
The mechanism by which ultraviolet rays cause sun-damaged skin has been extensively studied. Laboratory experiments indicate that the wavelengths with the most potential for carcinogenesis are those in the range of 280 to 320 nm, the ultraviolet B band. This ultraviolet B is responsible for the common sunburn. The transition from normal to actinic (i.e., sun damaged) to cancerous skin is usually a progressive process that occurs over several decades.
With the current environmental changes occurring with the earth's protective ozone layer, the concern for skin cancer becomes much more significant. A dramatic ozone depletion above the Antarctic continent has been detected. For each 1% reduction in atmospheric ozone concentration, there is a concomitant 2% increase in ultraviolet B penetration.
The carcinogenesis of epidermal tumors parallels the multistep development of other tumors. As with other tumors, certain characteristics render the host more susceptible to the development of cancer. Traits that are associated with an increased incidence of skin cancer include fair complexion, rays hair, blue or green eyes, inability to tan, propensity to sunburn, history of multiple or severe sunburns, and Celtic ancestry. Other factors implicated include age, occupation, habits (tanning booths), and residential geography, which are considered indirect causes of increased sun exposure.
The bulbs used in tanning booths are almost exclusively ultraviolet A wavelength and are promoted as providing a safe suntan. However, recent evidence indicates that ultraviolet A (320 to 400 nm) synergistically augments ultraviolet B responses and is independently capable of producing deleterious skin alterations and carcinogenesis.
Other etiologic factors are associated with the development of skin cancer. Chronic exposure to chemical agents, such as arsenic in patients treated with Fowlers solution, has been associated with the development of multiple squamous and basal cell tumors. Patients with chronic ra-diodermatitis, resulting from superficial Radiotherapy, demonstrate a propensity to develop multiple and aggressive lesions. Trauma in the form of burns, ulcers, and scars is also associated with the development of skin cancer (i.e., Marjolins ulcer). Immunosuppression, common in transplant patients and patients with leukemia or lymphoma, can be complicated by an increased incidence or aggressiveness of skin cancers .
Studies of human papilloma virus offer additional support for the importance of immune dysfunction in the development of skin squamous cell skin cancer. One study showed human papilloma virus presence in 60% of skin squamous cell skin cancer lesions found in renal autograft recipients. Moreover, this human papillomavirus presence was significantly higher than that found in matched transplant recipients without skin cancer. There also appears to be a high incidence of human papillomavirus in squamous cell skin cancer lesions of the cervix, penis, and digits.
Genetic syndromes, such as xeroderma pigmentosum (autosomal recessive) and nevoid basal cell skin cancer syndrome (autosomal dominant), are associated with a predilection for developing multiple basal cell skin cancers, often at an early age.
Thursday, June 5, 2008
 What is Basal cell carcinoma
What is Basal cell carcinoma
Basal cell carcinoma is the commonest malignant tumor affecting the skin. Clinically, it is a slow-growing locally invasive and locally destructive tumor in which distant metastases rarely occur. Several types are described based on their physical appearance, and there are a variety of clinical classifications. The essential component determined by clinical investigation is the extent of the tumor. Localized tumors generally have a clear cut-off point from tumor to normal tissue and the margins can be well defined. The papulonodular variety fits the classical description of the rodent ulcer with a rolled, pearly edge which often develops central ulceration. The solid type almost appears to grow out of the skin in an exophytic way and frequently has telangiatatic vessels coursing across its surface. The cystic variety can appear as a thin cyst, particularly around the eyelids, and sometimes also has telangiatatic vessels.
In the diffuse type, the pattern of spread is insidious, and defining the tumor margins can be difficult. The infiltrating type is clearly not purely an exophytic growth and infiltration of adjacent tissues can be demonstrated by palpation. The multifocal variety appears to have areas of almost normal looking skin which may represent healing of a previously ulcerated area. These multifocal lesions may be superficial or can infiltrate in depth. The morphoeic type of basal cell carcinoma infiltrates the dermis and produces a dense stromal reaction with stromal fibrosis. This gives the skin a characteristic white plaque-like appearance which is stiff, hence the term morphoea. The difficulty lies in determining the lateral extent of these tumors because of the dense stromal reaction. Metatypical basal cell carcinomas commonly appear as large, ulcerating, often exophytic, lesions. Characteristically they look very similar to squamous cell carcinomas but have a long history. It is the length of history that usually differentiates these tumors. Patients may present with more than one primary basal cell carcinoma which may be associated with a syndrome as discussed previously. Patients who have a basal cell carcinoma show a high incidence of a second lesion compared with the normal population. Second basal cell carcinomas frequently develop in the first year (16 per cent) and the incidence then falls to approximately 10 per cent over the next 4 years.
In addition to the physical appearance of primary basal cell carcinoma, there are three other clinical pictures which are encountered, namely recurrent basal cell carcinoma, aggressive or horrifying basal cell carcinoma, and metastatic basal cell carcinoma.
Recurrent basal cell carcinoma
The clinical appearance of recurrent basal cell carcinoma is very variable and is often dependent on previous treatment. The margins are difficult to determine but recurrence should always be regarded as having a diffuse pattern. Patients at risk of developing recurrence include those who have already presented with recurrent basal cell carcinoma, those with basal cell carcinomas showing an aggressive histological pattern, and lesions arising at cosmetically sensitive sites where tissue is scarce.
Horrifying basal cell carcinoma
This is the most dangerous basal cell carcinoma, characterized clinically by deep invasion and widespread destruction of adjacent tissues. The terminology is somewhat confusing, since it is also termed aggressive basal cell carcinoma, but this latter term applies to the histological appearance rather than the clinical appearance and behaviour. These tumors tend to occur in young individuals; they are large (more than 3 cm) and often appear in the region of the head and neck, particularly the scalp. Inadequate primary treatment is cited as an important factor in the development of horrifying basal cell carcinoma, particularly deep tumor extension following radiotherapy. The diffuse infiltrative type of basal cell carcinoma has been implicated in the development of these horrifying lesions, with a high incidence in morphoeic or metatypical basal cell carcinomas and those with adenoid differentiation. Unusual aetiologies such as arsenic, immuosuppression, and X-ray-induced tumors have also been implicated.
The clinical danger of these tumors, particularly those arising in the head and neck, is their capacity to infiltrate through bone and into the central nervous system causing widespread local destruction.
Metastatic basal cell carcinoma
The development of a distant metastasis from a basal cell carcinoma is exceptionally rare, so much so that it remains worthy of a single case report. The incidence is reported as varying from 0.0028 per cent to 0.55 per cent. Characteristically, patients have a large basal cell carcinoma, usually of long standing, which has proved resistant to treatment at the local site. Metatypical basal cell carcinoma with squamous differentiation has been associated with metastases. Facial basal cell carcinomas tend to metastasize to regional nodes, but a wide variety of organs have also been reported as showing metastasis including lung, liver, brain, heart, and pericardium. The histological variations that occur in basal cell carcinoma continue to fuel the debate as to whether basal cell carcinomas do indeed metastasize or whether these tumors are variants of adnexal tumors.
Pathological features
One of the major problems with basal cell carcinoma is that the cell of origin and the histiogenesis have not been accurately determined. The keratin phenotype of basal cell carcinoma would suggest an origin in the hair follicles. If this is indeed the case, then basal cell carcinoma is a type of adnexal tumor; however, because of its frequent occurrence it has been classified separately. Unlike many other tumors there does not appear to be a premalignant phase for basal cell carcinoma arising de novo. Basal cell carcinomas are composed of islands or nests of basophilic cells which resemble miniature basal cells of the epidermis lying in a connective tissue stroma. Typically the cells pack together in a regular manner to produce peripheral palisading. The cellular islands and nests within the stroma give them different patterns, and this has led to a variety of classifications. From the clinical perspective, it is the pattern and degree of infiltration and the arrangement of cells within the stroma that is most important rather than the degree of differentiation or number of mitoses present.